Working to Revolutionize Cancer Care Through Non-invasive Solutions
Cancer is a leading cause of death globally. Despite, decades of effort and aggressive cancer treatments, cancer remains a civilizational challenge.
Datar Cancer Genetics is a leader in developing blood-based diagnostics that can detect cancer early, offer non-invasive investigations for better treatment options, and track the progression of the disease or its recurrence.
We believe that relentless research, bold initiatives, sustained commitment, and an unapologetic determination to succeed against cancer will help us defeat it. We dedicate ourselves to this singular mission as if our life depends on it.
Breakthrough Technology
Welcome to the forefront of cutting-edge cancer detection and management – where innovation meets personalization. In the realm of contemporary cancer wellness, liquid biopsy has emerged as a pivotal player, reshaping the landscape of cancer screening and diagnostics. At the forefront of this revolution are our visionary scientists, who have devoted their expertise and ingenuity to craft unparalleled customized, non-invasive tests for the benefit of patients. By harnessing the power of molecular science and unraveling the mysteries held within Circulating Tumor Cells (CTCs) and DNA / RNA fragments, we have ushered in a new era of personalized cancer management through liquid biopsy. Our groundbreaking tests serve as the key to unlocking the potential of molecularly targeted therapies, providing a profound understanding of tumor heterogeneity and dynamic tumor biology. This enables us to devise optimum treatments even for the most difficult cases. We look forward to being of assistance to you in the fight against cancer with the unwavering commitment of all resources at our command.
The Revolutionary Changes
We Are Making in Cancer Care
Effect of Our Proprietary CEM
on Benign Cells, PBMCs
and Malignant Cells
Chemosensitivity Evaluation
of Circulating Tumor
Associated Cells
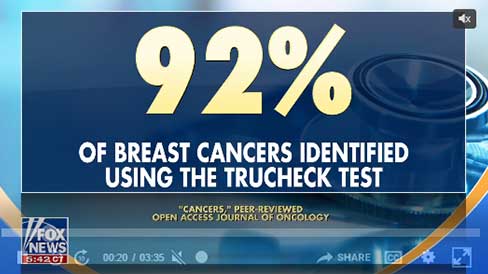
Studies Show Blood Tests
Reveal Breast Cancer
Earlier Than Mammograms
CEM = CTC Enrichment Medium
PBMC = Peripheral Blood Mononuclear Cells
Contact Us
10 Medawar Road,
The Surrey Research Park, Guildford,
Surrey, GU2 7AE, United Kingdom
Bayreuth 95447,
Germany
Nasik, Maharashtra.
India
Accreditation*

To see our NABL-accredited scope, please follow the links for NABL accredited Scope.
*India Lab